
Complexes
Reaction types
What is complexes?
The formation of complexes is an electrostatic effect, i.e. differences in charge distribution giving attraction or repulsion. The orientation of the units in the complex are just as fixed as what you see in covalent or ionic bonds. This is called coordination and the coordinated units are called ligands. For complexes there is no formal sharing of electrons, as seen with the covalent bonds. In reality this is not entirely correct, as there will be a general exchange of electrons, but as a model for description and calculation on complexes, it is a good approximation.
What do they look like?
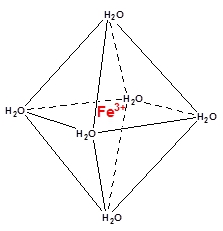 Around small, simple ions, you usually have six available positions for complexes, e.g. as the green iron(III) hexahydrate complex (see the structure to the right). However, the positions aren't necessarily being used. The spaces are filled out, usually by the solvent, if it is a solution, what whatever is in the positions isn't coordinated. The six positions are also under the condition that there is room for them. The ligands can easily be so big, that you have sterical limitations, i.e. the ligands are so big they get in each other's way. E.g. the complex [CuCl4]2- has the four Cl- in a tetrahedral formation, as an effect of the electrostatic forces (their repulsion of each other) and the size of Cl-.
Around small, simple ions, you usually have six available positions for complexes, e.g. as the green iron(III) hexahydrate complex (see the structure to the right). However, the positions aren't necessarily being used. The spaces are filled out, usually by the solvent, if it is a solution, what whatever is in the positions isn't coordinated. The six positions are also under the condition that there is room for them. The ligands can easily be so big, that you have sterical limitations, i.e. the ligands are so big they get in each other's way. E.g. the complex [CuCl4]2- has the four Cl- in a tetrahedral formation, as an effect of the electrostatic forces (their repulsion of each other) and the size of Cl-.Even though complexes contains a solvent, it doesn't have to be in solution. Crystal water is a common phenomenon in inorganic compounds. Copper(II) sulfate, CuSO4 is a white salt, without crystal water, and a blue salt as the pentahydrate, CuSO4 · 5 H2O.
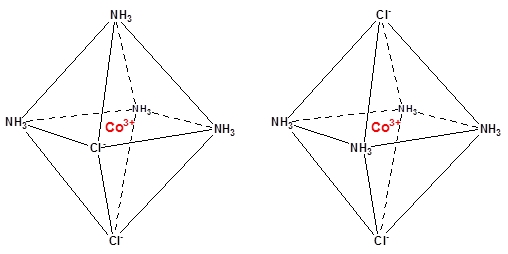
There may be geometric isomers in the complexes. The cobalt(III) chloride/ammonia complex, CoCl3(NH3)4, can be found as two isomers, with different colors. Of the three chloride, two of them are locked in position in the complex. Here they can be positioned cis or trans, i.e. next to each other or on opposite sides of the Co3+ (see the structures below). The trans complex is green, and the cis complex is purple.
Larger complexes like iodine/starch, used for iodometric titration, requires structure determination as seen with 2D NMR or x-ray crystallography.
Chemical equations
As reactions, complex formations aren't different from precipitations or equilibria, when it comes to balancing or calculations, e.g.
Silver ions and ammonia forming diamine silver complexes:
Ag+(aq) + 2 NH3(aq) [Ag(NH3)2]+(aq)
[Ag(NH3)2]+(aq)
Copper(II) ions forming green complexes with cyanide ions:
Cu2+(aq) + 4 CN-(aq) [Cu(CN)4]2-(aq)
[Cu(CN)4]2-(aq)
Silver ions and ammonia forming diamine silver complexes:
Ag+(aq) + 2 NH3(aq)
 [Ag(NH3)2]+(aq)
[Ag(NH3)2]+(aq)Copper(II) ions forming green complexes with cyanide ions:
Cu2+(aq) + 4 CN-(aq)
 [Cu(CN)4]2-(aq)
[Cu(CN)4]2-(aq)