
Resonance structures
Reaction types
What is resonance structures?
Usually we think of bonds as localized or static structures, and in most cases they act like they are, at least for basic descriptive purposes. In practical chemistry and advanced descriptions, resonance structures go a long way in describing chemical reactions or lack thereof.
The classical example in resonance structures is the benzene ring. Benzene is a ring of 6 carbon atoms with conjugated double bonds (i.e. alternating single and double bonds). When drawing the ring, the double bonds can be located in 2 different positions as shown in fig. 1. Both are equally right and wrong. The double bond between position 1 and 2 is there some of the time, but on average there is really 1.5 bonds between all the carbons. The bond is said to be delocalized, which is why the benzene ring is often written as a hexagon with a circle.
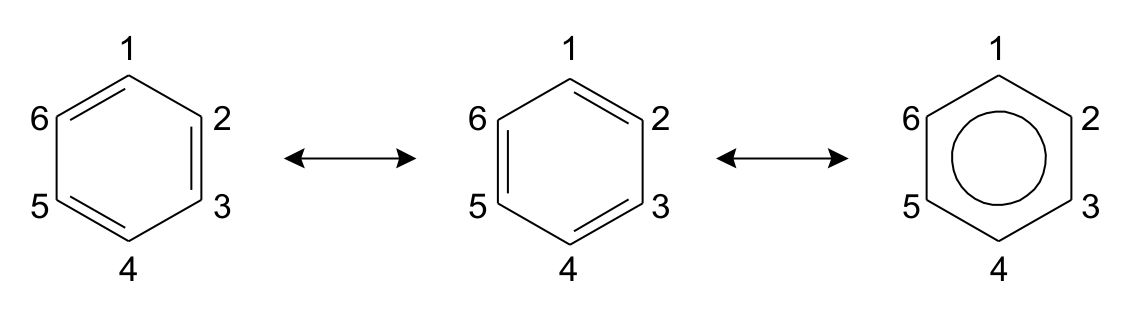
Fig. 1: Resonance structures in benzene.
The difference from rearranging the electrons is called resonance structures, and the change between structures is shown by a single arrow pointing both ways.
The classical example in resonance structures is the benzene ring. Benzene is a ring of 6 carbon atoms with conjugated double bonds (i.e. alternating single and double bonds). When drawing the ring, the double bonds can be located in 2 different positions as shown in fig. 1. Both are equally right and wrong. The double bond between position 1 and 2 is there some of the time, but on average there is really 1.5 bonds between all the carbons. The bond is said to be delocalized, which is why the benzene ring is often written as a hexagon with a circle.

Fig. 1: Resonance structures in benzene.
The difference from rearranging the electrons is called resonance structures, and the change between structures is shown by a single arrow pointing both ways.
What is the practical importance of resonance structures?
The resonance structures are important, as they explain several effects in organic synthesis like why the benzene structure is so stable and why some substitutions like amine on benzene directs ortho and para (positions 2, 4, and 6) while others like the nitro group direct meta (positions 3 and 5) in electrophilic aromatic substitution.
Especially the keton/enol isomers (fig. 2) are of importance, as the stability of the isomers affect the efficiency of the reaction in various addition and elimination reactions.
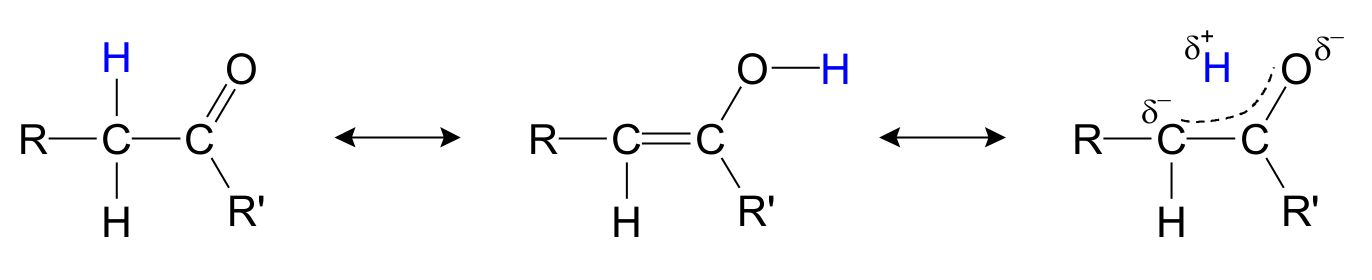
Fig. 2: Resonance structures for keton/enol.
As seen, the hydrogen marked with blue can be found next to the carbonyl group, which is the traditional 2D way of drawing a keton, but the hydrogen can also move over to the oxygen, forcing the formation of an alcohol group and a C-C double bond. The actual structure is for the most part in between with the hydrogen floating between the carbon and the oxygen and the double bond spread out between the C-C and the C-O bonds.
Especially the keton/enol isomers (fig. 2) are of importance, as the stability of the isomers affect the efficiency of the reaction in various addition and elimination reactions.

Fig. 2: Resonance structures for keton/enol.
As seen, the hydrogen marked with blue can be found next to the carbonyl group, which is the traditional 2D way of drawing a keton, but the hydrogen can also move over to the oxygen, forcing the formation of an alcohol group and a C-C double bond. The actual structure is for the most part in between with the hydrogen floating between the carbon and the oxygen and the double bond spread out between the C-C and the C-O bonds.